Frequently Accessed Pages
Effort Commitments and Payroll Certification
RAMP - Frequently Asked Questions (FAQ)
No-Cost Extension Request Procedures
Extramural Support Policies and Procedures
Research Education Development (RED)
Award Statistics FY23
Total Awards
$1928M
Federal Awards
$1125M
Non-Federal Awards
$803M
Research Expenditures
8th (FY22)
Single IRB Guidance for UW Research Administrators
Page Updated: May 18, 2022
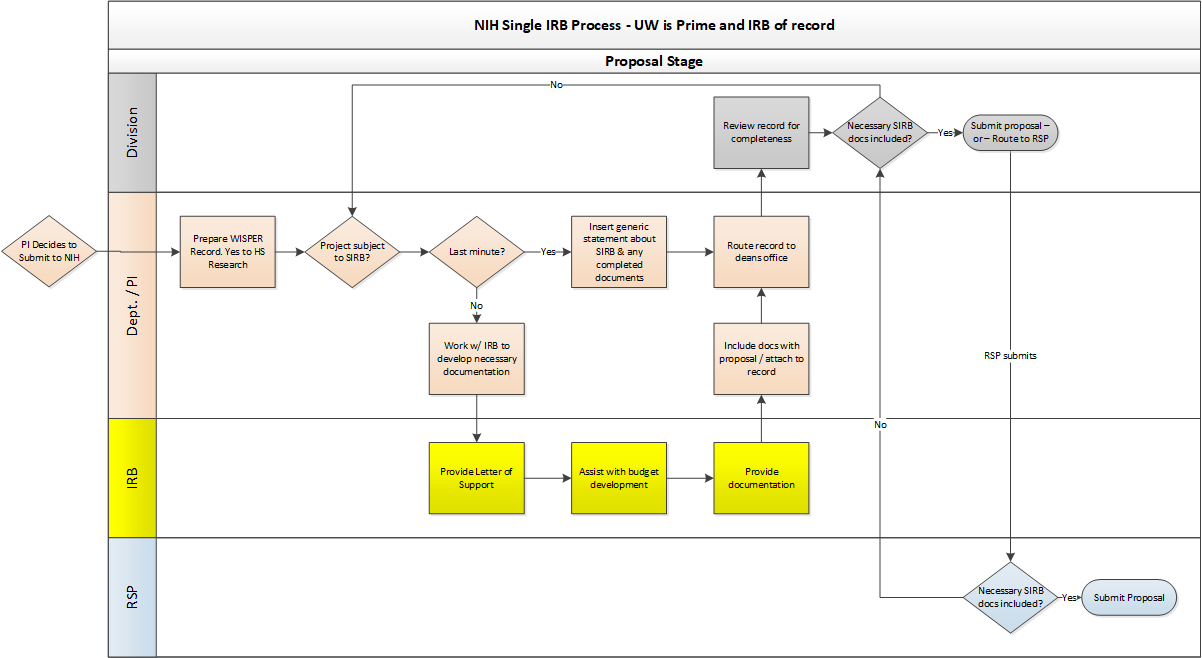
The purpose of this guidance is to assist Research Administrators at UW-Madison with the implementation of federal Single Institutional Review Board (SIRB) requirements to ensure proposals continue to be submitted on time and accurately.
Overview of Single IRB Requirements
Effective January 20, 2020, all federally funded multisite studies involving non-exempt human subjects research require single IRB review. The single IRB requirement is part of the revised Common Rule and applies to all federal agencies (e.g., NIH, Department of Education, NSF) and all types of non-exempt human subjects research, including social-behavioral studies.
Before submitting a proposal to a funding agency, study teams MUST consult with the Reliance and Navigation Team (RELIANT) for assistance in identifying the best single IRB of record for a study. Determining the best IRB option is based on factors such as study timeline, number of sites, and type of study. Single IRB options include UW-Madison, commercial IRBs, or other academic or healthcare institutions.
UW-Madison may agree to serve as the single IRB only for studies with external funding. See Appendices A and B of the reliance manual. Please note that UW-Madison cannot guarantee it will serve as the single IRB without prior consultation with RELIANT.
Study teams are encouraged to consult with RELIANT as early as possible in the proposal development stage to ensure single IRB requirements are addressed. To request a consultation, submit a request form or email irbreliance@wisc.edu for assistance.
Single IRB Plans
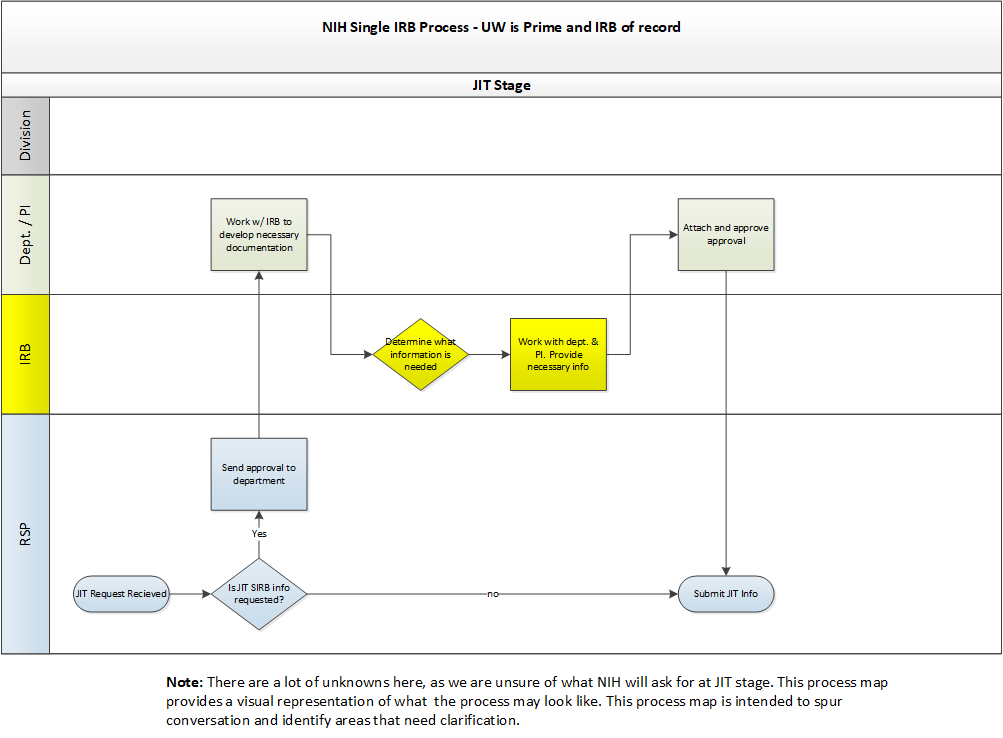
- NIH: Proposals must include a single IRB plan. Information about what should be addressed in the plan as well as NIH’s sIRB policy can be found here.
- A template single IRB plan is available by emailing RELIANT (irbreliance@wisc.edu).
- Other Agencies: A formal single IRB plan may not be required for proposals to non-NIH agencies. Study teams are encouraged to consult with their grants management specialist or program officer regarding what information about single IRB review should be included in their application.
Budget
- NIH: IRB fees can be included in proposal budgets as a direct cost. Information on budgeting for single IRB review costs can be found here.
- Other Agencies: IRB fees cannot be included in proposal budgets for studies not funded by NIH.
IRB Fees
- UW-Madison: UW-Madison does not charge IRB fees when serving as the single IRB. NOTE: If a commercial IRB will serve as the single IRB, a one-time administrative fee will be charged by UW-Madison.
- Commercial IRBs: UW-Madison has agreements with WCG and Advarra. Each has its own IRB fee schedule. For more information on fees charged by commercial IRBs, contact WCG and Advarra directly.
- Other Academic Institutions: Some academic institutions charge IRB fees when serving as the single IRB. This information is typically available on an IRB’s website.
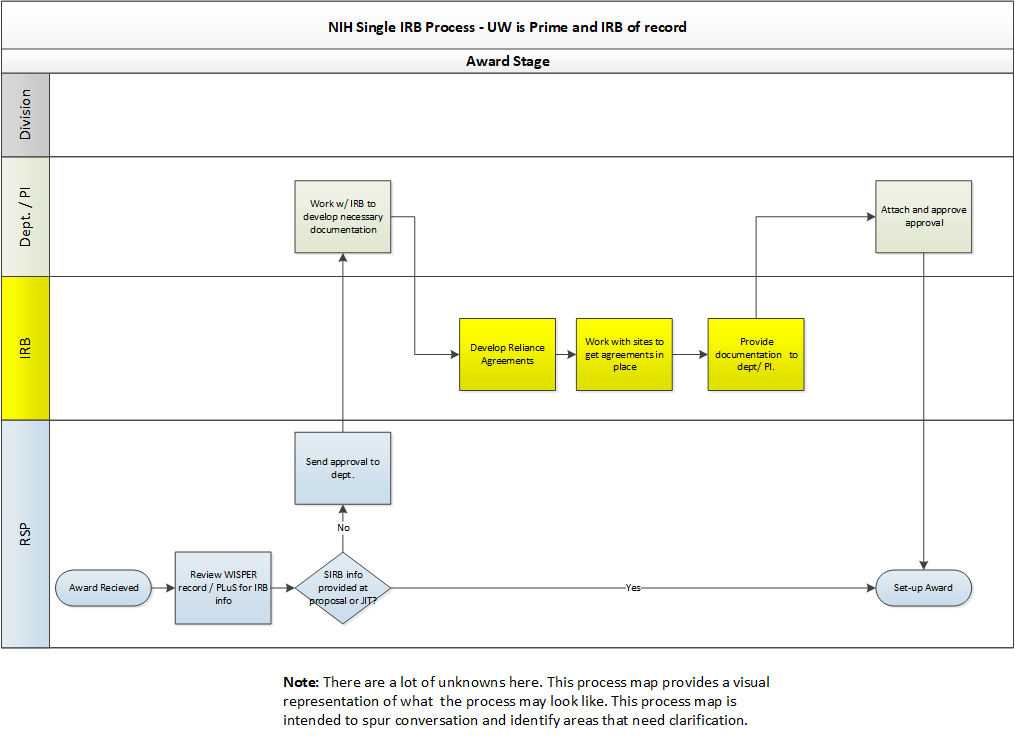
What Are the Next Steps for Single IRB Review After Funding Is Awarded?
When you receive notice that funding will be awarded, contact irbreliance@wisc.edu as soon as possible for assistance with single IRB arrangements. Please note that all reliance agreements (e.g., IRB authorization agreements) must be routed through the Reliance and Navigation Team (RELIANT) and signed by the UW’s institutional official.